Colonic Propulsive Motility Model
Discover how Melior’s unique phenotypic screening platforms can uncover the untapped value of your candidate therapeutic
This bead expulsion model is a method for evaluating a test compounds’ ability to alter colonic propulsive motility.
This model is designed to identify pharmacological agents that produce effects on propulsive motor activity and is an excellent predictor of drugs that may be useful for the therapy of irritable bowel syndrome (IBS) or constipation. This model can be modified to identify antagonists of constipative drugs or diarrhea producing drugs.
In this validation study, colonic propulsion was measured in mice treated with morphine, a well-described compound that reduces colonic motility.
This model is 1 of more than 60 models included in our theraTRACE® platform
Ready to get started or looking for a custom model?
Contact us today for more information about our bespoke research models and to discuss how we can help you answer your unique research questions.
Interested in running an Insulin Tolerance Test study?
If you are interested in learning more about the Insulin Tolerance Test (ITT), please contact us to start the conversation today!
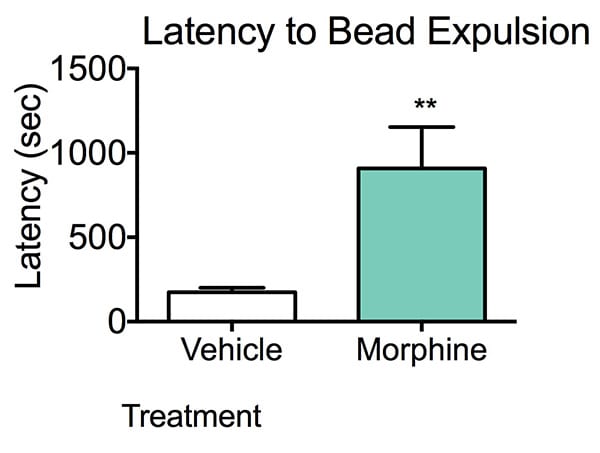
Latency to bead expulsion. CD1 mice (N=10) were injected with either vehicle or Morphine subcutaneously and thirty minutes later a 3mm glass bead is inserted into the distal colon of a mouse. Mice were monitored for up to thirty minutes and latency to bead expulsion was recorded. Morphine treated mice displayed a significantly higher latency to bead expulsion compared to vehicle treated animals. Data are mean ± SEM and evaluated using unpaired t-test; **p<0.01 compared to vehicle.
Morphine-treated animals can be used to screen antagonists of morphine-induced constipation, which is problematic in patients with extending treatment duration. This study validates the colonic expulsion assay and demonstrates that morphine, a well-described inhibitor of colonic propulsion, significantly attenuated propulsive activity.
This model is typically used to evaluate acute effects of test articles (after single administration). It exhibits relatively low variability typically yielding highly significant effects with groups sizes of 6 to 8 animals.





