Fecal Pellet Output
Discover how Melior’s unique phenotypic screening platforms can uncover the untapped value of your candidate therapeutic
A simple and noninvasive means of evaluating gastrointestinal function involves measuring fecal pellet output over a given time period(s). This can involve not only pellet counts but also measuring total wet weight, total dry weight and descriptive observations. In turn these measures can be used to calculate average weight / pellet and wet versus dry ratio which provides a measure of moisture content while also serving as a colonic transit test.
This model is 1 of more than 60 models included in our theraTRACE® platform
Ready to get started or looking for a custom model?
Contact us today for more information about our bespoke research models and to discuss how we can help you answer your unique research questions.
The study illustrated below shows how the inhibition of colonic motility by morphine results in reduced fecal pellet output
Interested in running an Insulin Tolerance Test study?
If you are interested in learning more about the Insulin Tolerance Test (ITT), please contact us to start the conversation today!
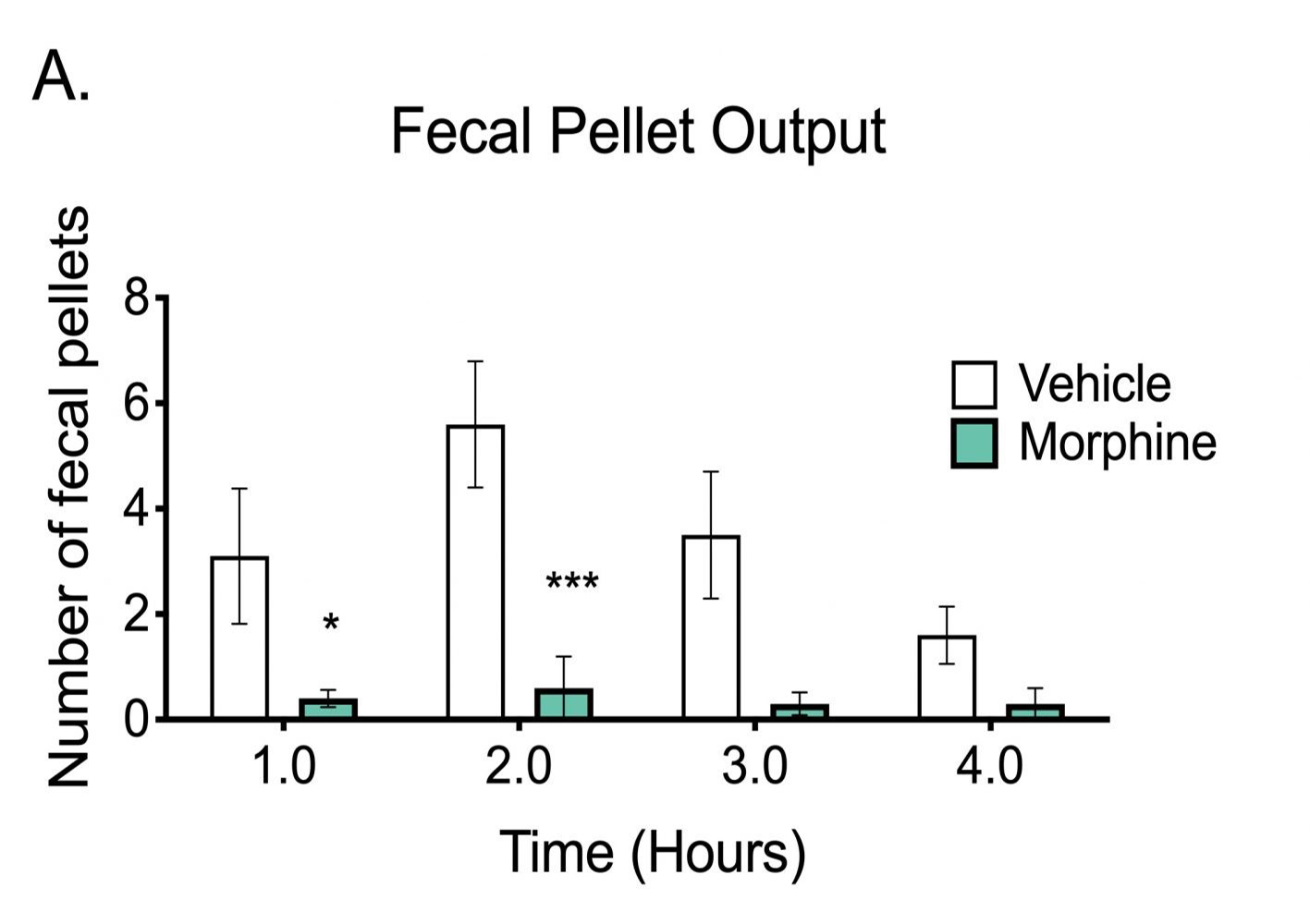
Fecal pellet output as a colonic transit test. C57Bl/6 mice were fed ad libitum until the acclimation period at approximately 9:00 AM. Then mice were weighed, dosed with vehicle or 10 mg/kg morphine SC, singly housed in polyurethane cages without access to food, and acclimated to the procedure room for 30 minutes prior to the study, with free access to water. With a study start at approximately 9:30 AM, at each subsequent hour after administration up to 4 hours, the number of fecal pellets (A) and weights (B) was recorded. Data are mean ± SEM and evaluated using unpaired t-test (N=10). *p<0.05, **p<0.01, ***p<0.001.






 Interested in running a Fecal Pellet Output study?
Interested in running a Fecal Pellet Output study?