The Diet-Induced Obesity (DIO) Mouse Model
A Model that Mirrors the Real-World Progression of Obesity
Unlike genetic obesity models, the DIO mouse model provides a nutritionally induced approach, making it an effective tool for studying environmental and lifestyle-driven obesity. The controlled high-fat diet protocol allows you to simulate real-world metabolic changes, including shifts in adiposity, glucose regulation, and lipid metabolism. Because obesity develops over time, this model captures both early metabolic adaptations and long-term disease progression, providing a more physiologically relevant platform for therapeutic evaluation.
With over two decades of experience, Melior has been at the forefront of metabolic disease research, collaborating with leading pharmaceutical companies and academic institutions to refine preclinical models. This expertise ensures that your DIO studies are rigorously designed, generating highly translatable insights for obesity-driven conditions such as type 2 diabetes, cardiovascular disease, and inflammatory disorders.
Advantages of the Diet-Induced Obesity Model
- Track adiposity and muscle mass over time with dual-energy x-ray absorptiometry (DEXA) imaging
- Reproducibly develop metabolic dysfunction under high-fat diet conditions
- Generate more physiologically relevant data than genetic models for obesity
- Capture early metabolic changes and long-term disease progression
This model is included in our theraTRACE® platform
We can customize your obesity study
Tailor your study with bespoke protocols, including DEXA imaging for body composition analysis, food intake monitoring, blood chemistry, glucose tolerance tests, and insulin sensitivity assessments.
Expand disease indications in the context of diet-induced obesity, including aging, inflammation, and psychiatric endpoints such as depression.
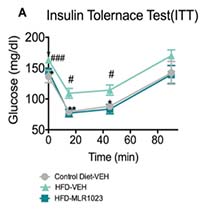
Interested in running an Insulin Tolerance Test study?
The Insulin Tolerance Test (ITT) is a key tool for measuring insulin sensitivity and glucose metabolism in preclinical models. This test provides critical insights into metabolic dysfunction and the efficacy of anti-diabetic therapies.

Fat and Lean Mass and percentage changes. DEXA analysis was performed at Baseline and on Weeks 2 and 4. All mice shared comparable fat/lean mass and fat percentage at the baseline. Compared to the mice of the DIO+Vehicle group, the mice of the DIO+SEMA group showed significantly reduced fat mass and fat percentage at weeks 2 and 4. Values show mean ±SEM (n=11 for DIO+SEMA, N=5 for DIO+Vehicle). Data were analyzed by 1-way ANOVA with Dunnett’s MCT with comparison to the DIO+Vehicle group. *P<0.05, **P<0.01, *** P<0.001, ****P<0.0001.
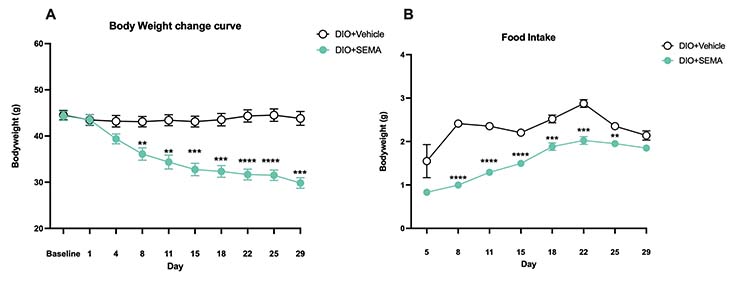
Body weights and food intake. Animals were weighed, and food intake was captured twice per week for the duration of the study. Compared to the DIO+Vehicle group, the mice of the DIO+SEMA group showed significant bodyweight reductions from Day 8 until the end of the study and reduced food consumption from day 8 until day 25. Values show mean ±SEM (n=11for DIO+SEMA, n=5 for DIO+Vehicle). Data were analyzed by 2-way ANOVA with Dunnett’s MCT.
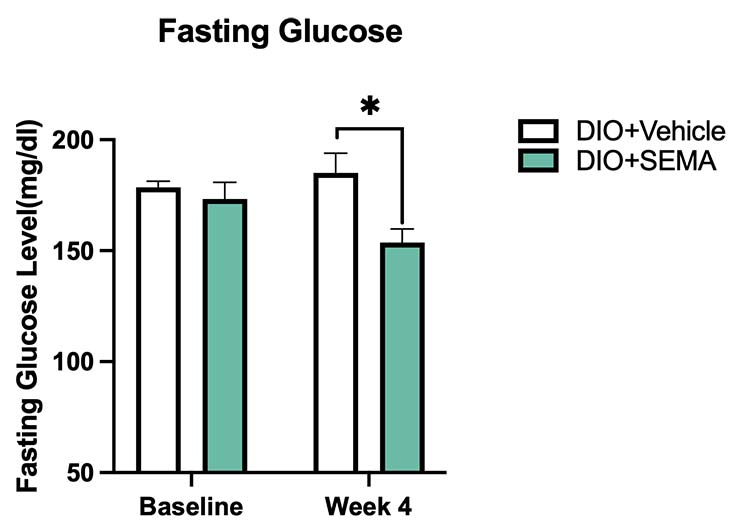
Fasting Blood Glucose. Fasting glucose was measured at Baseline and on Week 4. The SEMA-treated group exhibited significantly reduced fasting glucose levels after 4 weeks of treatment compared to the DIO+Vehicle group. Values show mean ±SEM (n=11 for DIO+SEMA, N=5 for DIO+Vehicle). Data were analyzed by 1-way ANOVA with Dunnett’s MCT. *P<0.05.
Publications
Frequently Asked Questions
To induce obesity, mice are typically placed on a high-fat diet for 10–16 weeks beginning at 6 weeks of age.
Studies evaluating GLP-1 analogs or other interventions can range from 2–6 weeks, but based on research needs, durations from 1 day to several months are possible.
This DIO model supports a range of metabolic assessments to evaluate glucose metabolism, insulin function, and energy balance, including:
- DEXA imaging for body composition analysis (adiposity and muscle mass)
- Food intake monitoring
- Fasting glucose measurements for baseline metabolic status
- Insulin Tolerance Test (ITT)
- Oral Glucose Tolerance Test (GTT)
- Hyperinsulinemic-euglycemic clamp for precise insulin function analysis
- Blood chemistry analysis for glucose and lipid metabolism markers
As well as non-metabolic assessments, including:
- Histology
- Inflammatory markers
- Psychiatric endpoints (e.g., depression-related behavioral tests)
Yes! While commonly used for obesity, diabetes, and metabolic syndrome, our DIO mouse model can also support research into aging, inflammation, and psychiatric conditions such as depression, providing a broader view of obesity’s systemic effects.
Citations
Lang, P., Hasselwander, S., Li, H., & Xia, N. (2019). Effects of different diets used in diet-induced obesity models on insulin resistance and vascular dysfunction in C57BL/6 mice. Scientific reports, 9(1), 19556. https://doi.org/10.1038/s41598-019-55987-x
Li, Y., Li, X., Xue, Q., Wang, J., & Tan, J. (2021). High-fat diet and dyslipidemia synergistically contribute to T cell senescence in gut associated lymphoid tissue. Experimental gerontology, 151, 111404. https://doi.org/10.1016/j.exger.2021.111404





